![Depict high spin and low spin configurations for each of the following complexes. Tell whether each is diamagnetic or paramagnetic. Give the number of unpaired electrons of the paramagnetic complexes: [Fe(CN)6]^4 - Depict high spin and low spin configurations for each of the following complexes. Tell whether each is diamagnetic or paramagnetic. Give the number of unpaired electrons of the paramagnetic complexes: [Fe(CN)6]^4 -](https://haygot.s3.amazonaws.com/questions/1573946_1731669_ans_b9791331ab454ed5935dfa969df3bda9.jpg)
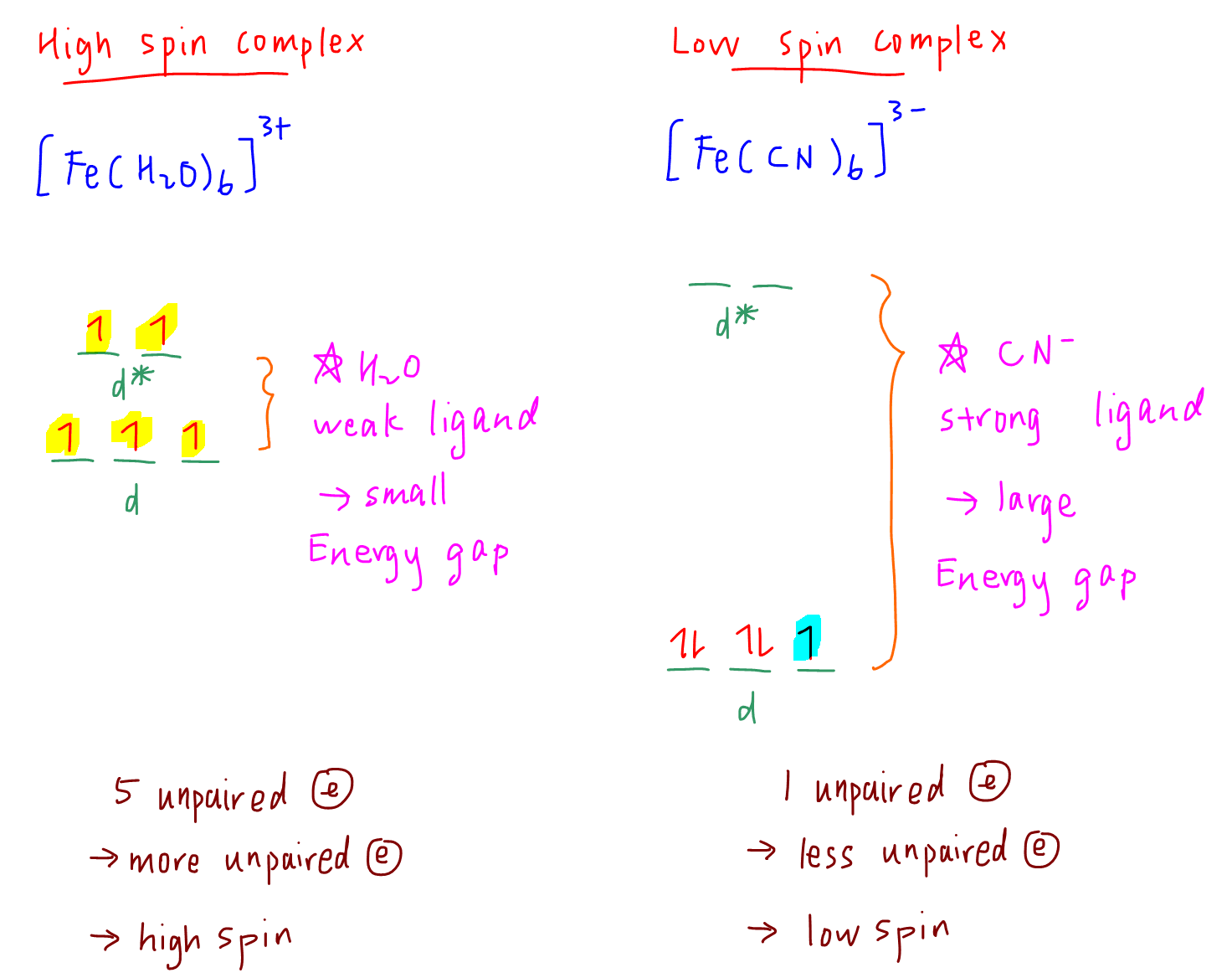
Depict high spin and low spin configurations for each of the following complexes. Tell whether each is diamagnetic or paramagnetic. Give the number of unpaired electrons of the paramagnetic complexes: [Fe(CN)6]^4 -

High-spin and low-spin states of spin-crossover molecules as revealed... | Download Scientific Diagram

Table 2 from High-spin and low-spin iron(II) complexes with facially-coordinated borohydride ligands. | Semantic Scholar
Which transition metal can form both a high and low spin complex? "Zn"^(2+), "Cu"^(2+), "Mn"^(3+), "Ti"^(2+) | Socratic

Example of high-spin (S = 5/2) and low spin (S = 1/2) configurations... | Download Scientific Diagram

Explain why Cr^(2+) forms high-spin and low-spin octahedral complexes, but Cr^(3+) does not. | Homework.Study.com

Electronic diagram of the high-spin (HS) and low-spin (LS) states for a... | Download Scientific Diagram
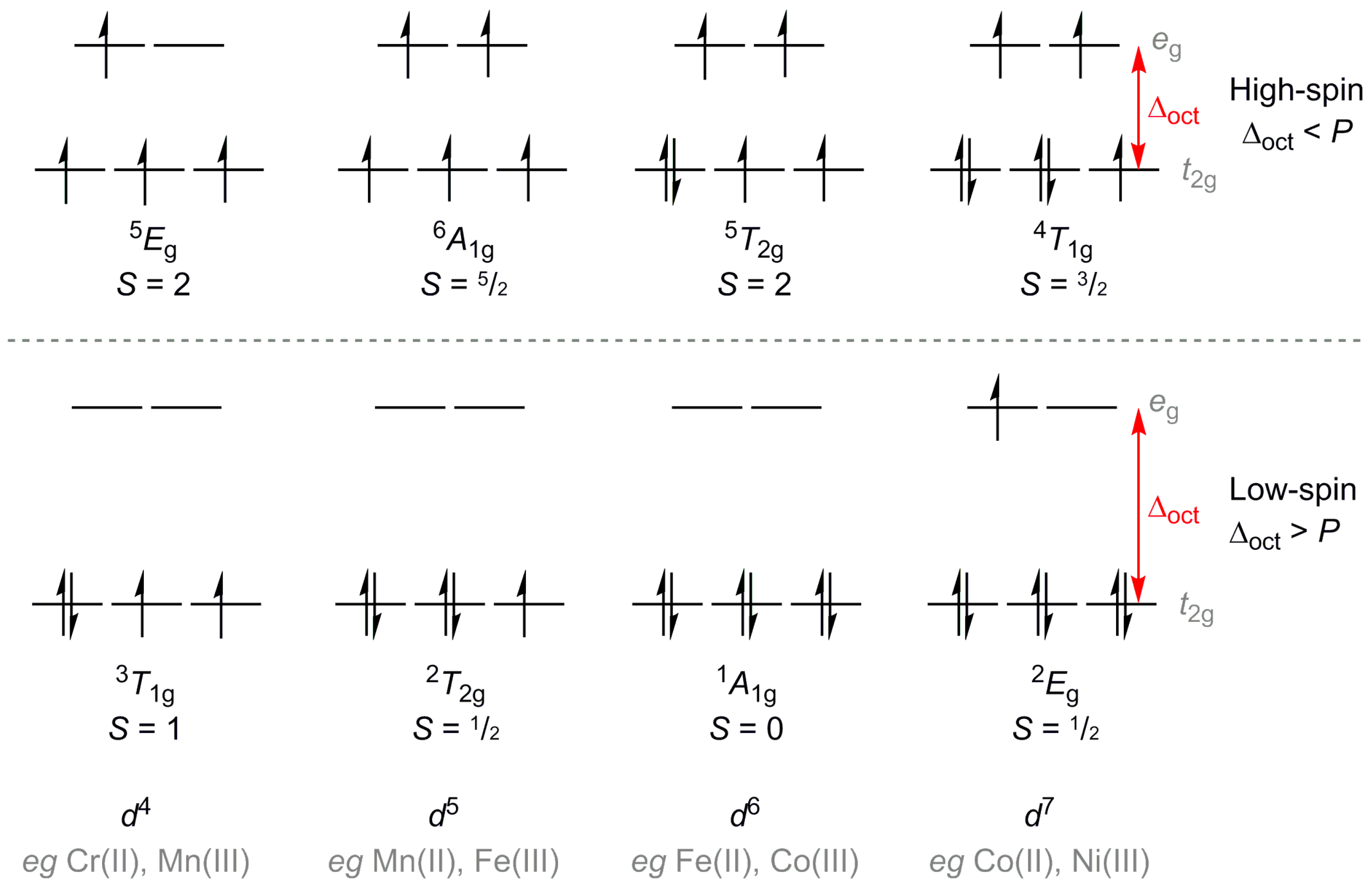
Crystals | Free Full-Text | The Effect of Ligand Design on Metal Ion Spin State—Lessons from Spin Crossover Complexes

a. Describe the steps in the formation of a high-spin octahedral complex of Fe^2+ in valence bond terms. b. Do the same for a low-spin complex. | Homework.Study.com
![Justify the formation of low spin complex and high spin comples taking the examples of [Fe(CN)6]3- and [FeF6]3- on the - Chemistry - Coordination Compounds - 12492103 | Meritnation.com Justify the formation of low spin complex and high spin comples taking the examples of [Fe(CN)6]3- and [FeF6]3- on the - Chemistry - Coordination Compounds - 12492103 | Meritnation.com](https://s3mn.mnimgs.com/img/shared/content_ck_images/ck_5aced292a812c.png)
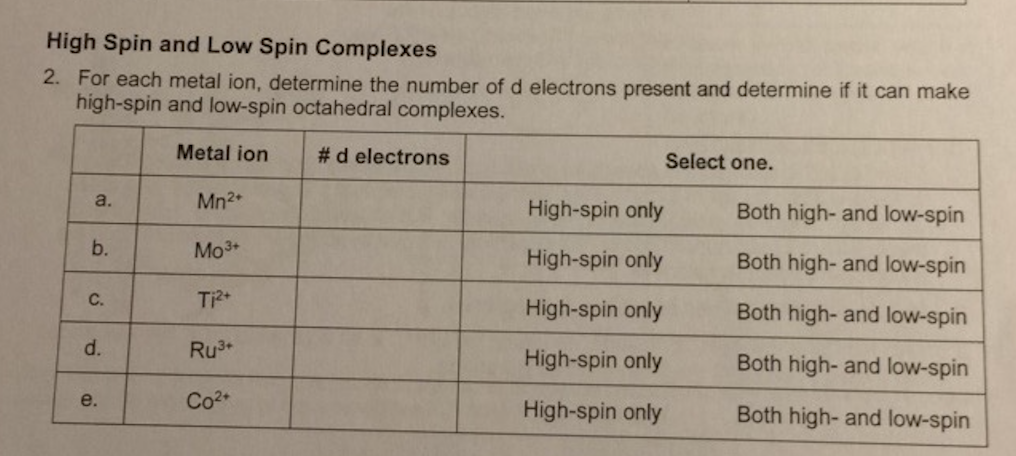


![Why is [math][Cr (NH_3)] ^ {3+}[/math] a high spin complex? - Quora Why is [math][Cr (NH_3)] ^ {3+}[/math] a high spin complex? - Quora](https://qph.cf2.quoracdn.net/main-qimg-39c6ab9fa9f76d4aeb65987cf0c19533.webp)