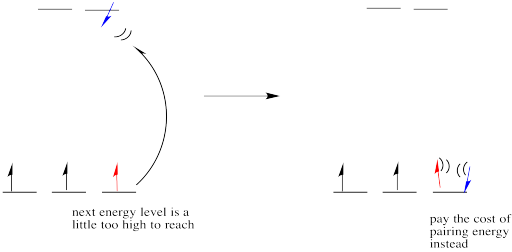
The sum of the crystal field stabilisation energy of high spin and low spin d 6 metal ion in octahedral field is calculated as x Δ0. The value of | x |=

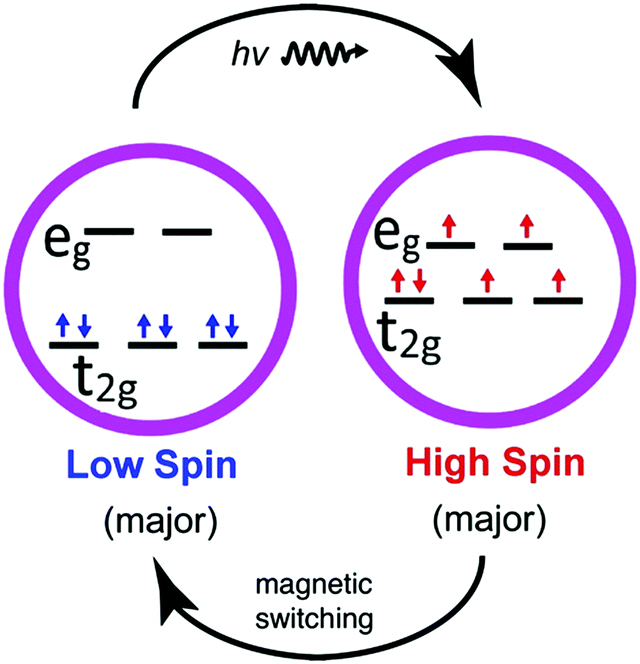
High-spin and low-spin states of spin-crossover molecules as revealed... | Download Scientific Diagram
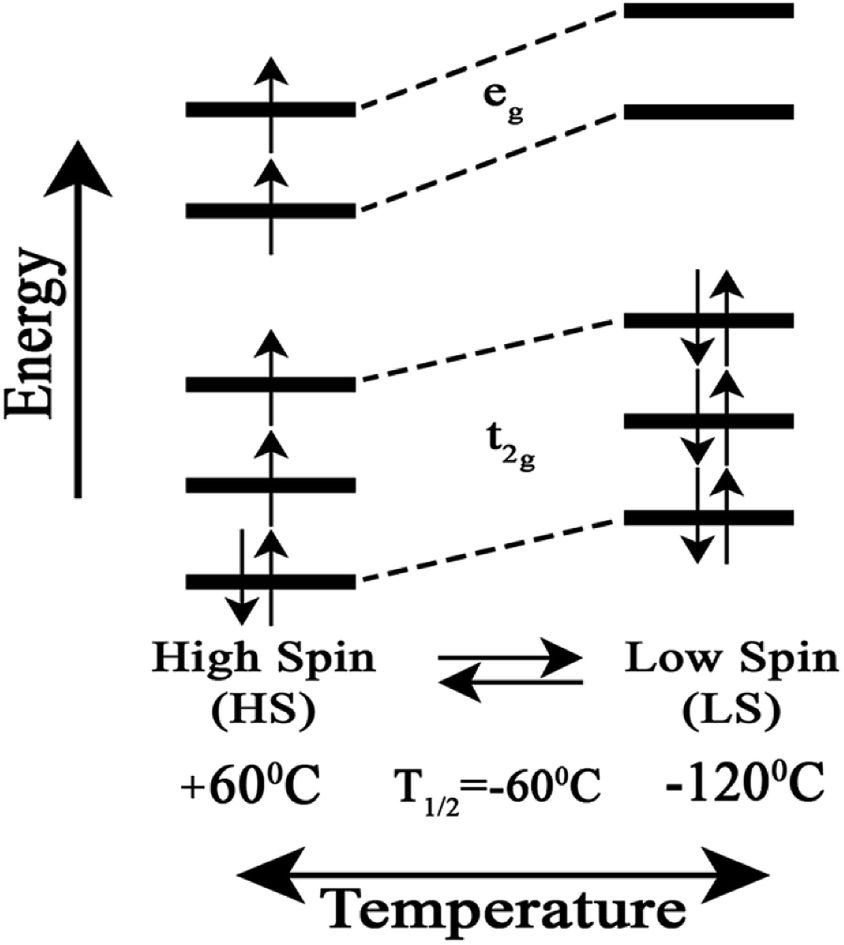
Reversible temperature-dependent high- to low-spin transition in the heme Fe–Cu binuclear center of cytochrome ba 3 oxidase - RSC Advances (RSC Publishing) DOI:10.1039/C8RA09954E

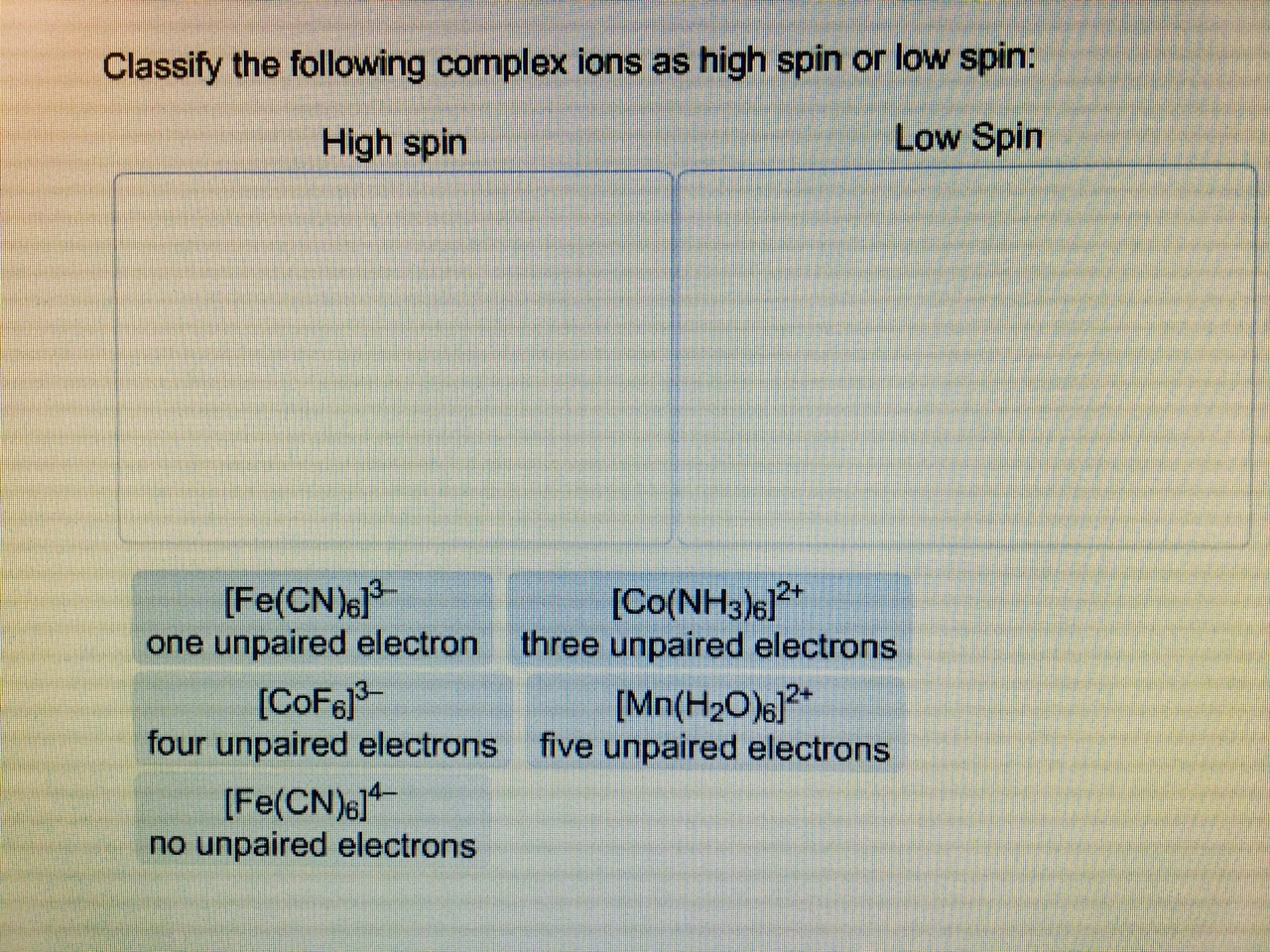
A metal ion in a high-spin octahedral complex has two more unpaired electrons compared to the same ion in a low-spin octahedral complex. Name some possible metal ions for which this arrangement
![Depict high spin and low spin configurations for each of the following complexes. Tell whether each is diamagnetic or paramagnetic. Give the number of unpaired electrons of the paramagnetic complexes: [Fe(CN)6]^4 - Depict high spin and low spin configurations for each of the following complexes. Tell whether each is diamagnetic or paramagnetic. Give the number of unpaired electrons of the paramagnetic complexes: [Fe(CN)6]^4 -](https://haygot.s3.amazonaws.com/questions/1573946_1731669_ans_b9791331ab454ed5935dfa969df3bda9.jpg)
Depict high spin and low spin configurations for each of the following complexes. Tell whether each is diamagnetic or paramagnetic. Give the number of unpaired electrons of the paramagnetic complexes: [Fe(CN)6]^4 -